- Home ·
- Publications ·
- Research Area F ·
- 2007 ·
Active gamma-secretase complexes contain only one of each component
10-Aug-2007
Toru Sato, Thekla S. Diehl, Saravanakumar Narayanan, Satoru Funamoto, Yasuo Ihara, Bart De Strooper, Harald Steiner, Christian Haass, and Michael S. Wolfe
J. Biol. Chem., 2007, doi:10.1074/jbc.M705248200, published on 02.10.2007
The Journal of Biological Chemistry, online article
The Journal of Biological Chemistry, online article
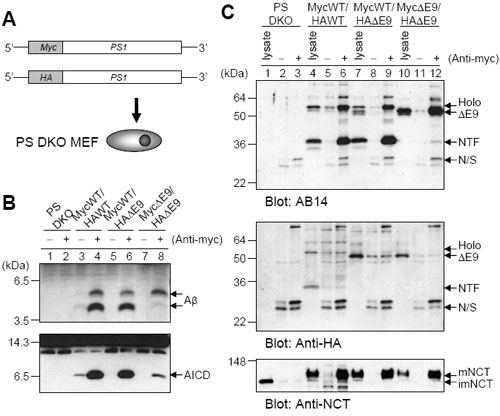
gamma-Secretase is an intramembrane aspartyl protease complex that cleaves type I integral membrane proteins, including the amyloid beta-protein precursor (APP) and the Notch receptor, and is composed of presenilin, Pen-2, nicastrin and Aph-1. Although all four of these membrane proteins are essential for assembly and activity, the stoichiometry of the complex is unknown, with the number of presenilin molecules present being especially controversial. Here we analyze functional gamma-secretase complexes, isolated by immunoprecipitation from solubilized membrane fractions and able to produce amyloid beta-peptides and APP intracellular domain. We show that the active isolated protease contains only one presenilin per complex, which excludes certain models of the active site that require aspartate dyads formed between two presenilin molecules. We also quantified components in the isolated complexes by western blot using protein standards, and found that the amounts of Pen-2 and nicastrin were the same as that of presenilin. Moreover, we found that one Aph-1 was not co-immunoprecipitated with another in active complexes, evidence that Aph-1 is likewise present as a monomer. Taken together, these results demonstrate that the stoichiometry of gamma-components presenilin : Pen-2 : nicastrin : Aph-1 is 1 : 1 : 1 : 1.