ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import
06-Jul-2010
The EMBO Journal, 2010, 29, 2841 - 57 published on 06.07.2010
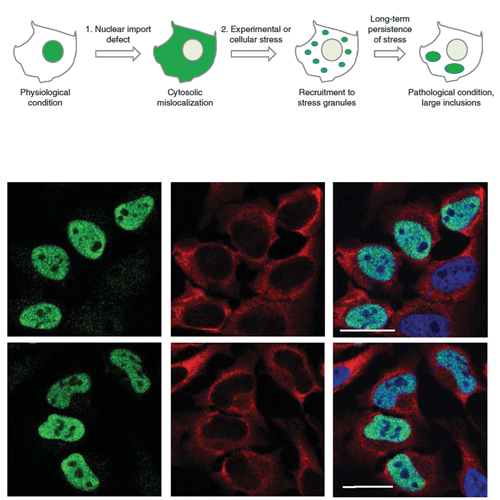
Mutations in fused in sarcoma (FUS) are a cause of familial amyotrophic lateral sclerosis (fALS). Patients carrying point mutations in the C-terminus of FUS show neuronal cytoplasmic FUS-positive inclusions, whereas in healthy controls, FUS is predominantly nuclear. Cytoplasmic FUS inclusions have also been identified in a subset of frontotemporal lobar degeneration (FTLD-FUS). We show that a non-classical PY nuclear localization signal (NLS) in the C-terminus of FUS is necessary for nuclear import. The majority of fALS-associated mutations occur within the NLS and impair nuclear import to a degree that correlates with the age of disease onset. This presents the first case of disease-causing mutations within a PY-NLS. Nuclear import of FUS is dependent on Transportin, and interference with this transport pathway leads to cytoplasmic redistribution and recruitment of FUS into stress granules. Moreover, proteins known to be stress granule markers co-deposit with inclusions in fALS and FTLD-FUS patients, implicating stress granule formation in the pathogenesis of these diseases. We propose that two pathological hits, namely nuclear import defects and cellular stress, are involved in the pathogenesis of FUS-opathies.