Beta-amyloid precursor protein mutants respond to gamma-secretase modulators
26-Mar-2010
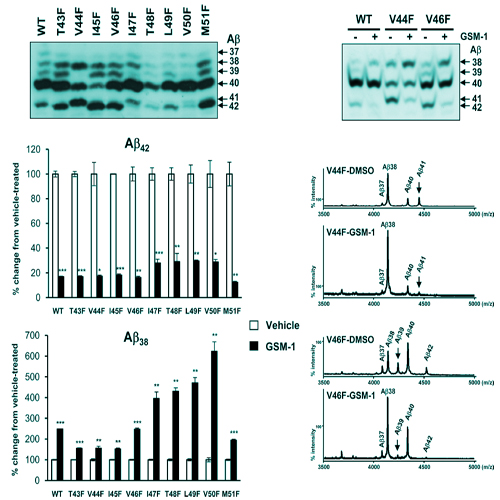
Pathogenic generation of the 42 amino acid variant of the amyloid beta-peptide (Abeta42) by beta- and gamma-secretase cleavage of the beta-amyloid precursor protein (APP) is believed to be causative for Alzheimer 's disease (AD). Lowering of Abeta42 production by gamma-secretase modulators (GSMs) is a hopeful approach towards AD treatment. The mechanism of GSM action is not fully understood. Moreover, whether GSMs target the Abeta domain is controversial. To further our understanding of the mode of action of GSMs and the cleavage mechanism of gamma-secretase, we analyzed mutations located at different positions of the APP TMD around or within the Abeta domain regarding their response to GSMs. We found that Abeta42-increasing familial AD (FAD) mutations of the gamma-secretase cleavage site domain responded robustly to Abeta42-lowering GSMs, especially to the potent compound GSM-1, irrespective of the amount of Abeta42 produced. We thus expect that FAD patients carrying mutations at the gamma-secretase cleavage sites of APP should respond to GSM-based therapeutic approaches. Systematic phenylalanine scanning mutagenesis of this region revealed a high permissiveness to GSM-1 and demonstrated a complex mechanism of GSM action as also other Abeta species (Abeta41, Abeta39) could be lowered besides Abeta42. Moreover, certain mutations simultaneously increased Abeta42 and the shorter peptide Abeta38 arguing that the proposed precursor-product relationship of these Abeta species is not general. Finally, mutations of residues in the proposed GSM-binding site implicated in Abeta42 generation (G29, G33) and potentially in GSM-binding (K28) were also responsive to GSMs, a finding that may question APP substrate targeting of GSMs.