Inhibition of mitochondrial fusion by Alpha-synuclein is rescued by PINK1 Parkin and DJ-1
20-Oct-2010
The EMBO Journal, 2010, 1-19, doi:10.1038/emboj.2010.223 published on 20.10.2010
The Embo Journal, online article
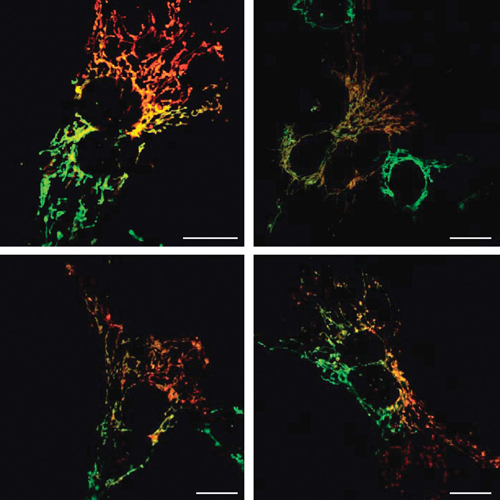
Aggregation of alpha-synuclein (alphaS) is involved in the pathogenesis of Parkinson’s disease (PD) and a variety of related neurodegenerative disorders. The physiological function of alphaS is largely unknown. We demonstrate with in vitro vesicle fusion experiments that alphaS has an inhibitory function on membrane fusion. Upon increased expression in cultured cells and in Caenorhabditis elegans, alphaS binds to mitochondria and leads to mitochondrial fragmentation. In C. elegans age-dependent fragmentation of mitochondria is enhanced and shifted to an earlier time point upon expression of exogenous alphaS. In contrast, siRNA-mediated downregulation of alphaS results in elongated mitochondria in cell culture. AlphaS can act independently of mitochondrial fusion and fission proteins in shifting the dynamic morphologic equilibrium of mitochondria towards reduced fusion. Upon cellular fusion, alphaS prevents fusion of differently labelled mitochondrial populations. Thus, alphaS inhibits fusion due to its unique membrane interaction. Finally, mitochondrial fragmentation induced by expression of alphaS is rescued by coexpression of PINK1, parkin or DJ-1 but not the PD-associated mutations PINK1 G309D and parkin D1–79 or by DJ-1 C106A.