- Home ·
- Publications ·
- Research Area F ·
- 2008 ·
Intramembrane Proteolysis by gamma-Secretase
23-Jul-2008
Harald Steiner, Regina Fluhrer, Christian Haass
Journal of Biological Chemistry, 2008, DOI 10.1074/jbc.R800010200, published on 23.07.2008
www.jbc.org, online article
www.jbc.org, online article
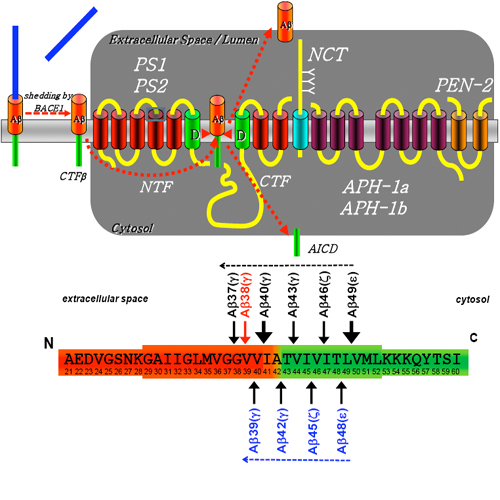
Gamma-Secretase mediates the final proteolytic cleavage, which liberates Amyloid beta -peptide, the major component of senile plaques in the brain of Alzheimer's disease patients. Therefore gamma -secretase is a prime target for Amyloid beta -peptide lowering therapeutic strategies. gamma -Secretase is a protein complex composed of four different subunits, presenilin, APH-1, NCT and PEN-2, which are most likely present in a 1:1:1:1 stoichiometry. Presenilin harbors the catalytically active site, which is critically required for the aspartyl protease activity of gamma -secretase. Moreover, numerous familial Alzheimer's disease associated mutations within the presenilins increase the production of the aggregation prone and neurotoxic 42 amino acid amyloid beta -peptide. NCT may serve as a substrate receptor, although this has recently been challenged. PEN-2 is required to stabilize presenilin within the gamma -secretase complex. No particular function has so far been assigned to APH-1. The four components are sufficient and required for gamma -secretase activity. At least six different gamma -secretase complexes exist which are composed of different variants of presenilin and APH-1. All gamma -secretase complexes can exert pathological amyloid beta -peptide production. Assembly of the gamma -secretase complex occurs within the endoplasmic reticulum and only fully assembled and functional gamma -secretase complexes are transported to the plasma membrane. Structural analysis by electron microscopy and chemical cross-linking reveals a water-containing cavity, which allows intramembrane proteolysis. Specific and highly sensitive gamma -secretase inhibitors have been developed, however they interfere with the physiological function of gamma -secretase in Notch-signaling and thus cause rather significant side effects in human trials. Modulators of gamma -secretase, which selectively affect the production of the pathological 42 amino acid amyloid beta -peptide do not inhibit Notch signaling.