Reactivation of the Same Synapses during Spontaneous Up States and Sensory Stimuli
27-Jun-2013
Cell Reports, 2013, doi: 10.1016/j.celrep.2013.05.042, Volume 4, Issue 1, 31-39 published on 27.06.2013
Cell Reports, online article
Cell Reports, online article
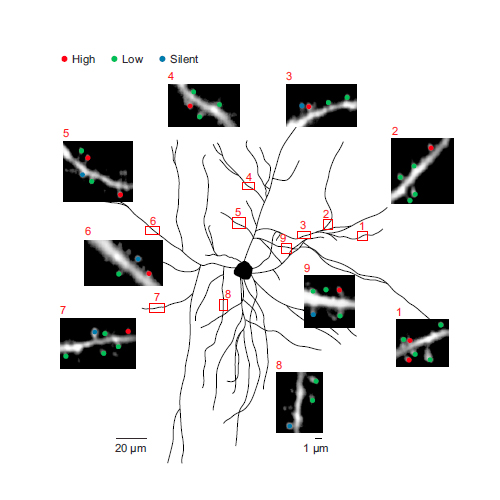
In the mammalian brain, calcium signals in dendritic spines are involved in many neuronal functions, particularly in the induction of synaptic plasticity. Recent studies have identified sensory stimulation-evoked spine calcium signals in cortical neurons in vivo. However, spine signaling during ongoing cortical activity in the absence of sensory input, which is essential for important functions like memory consolidation, is not well understood. Here, by using in vivo two-photon imaging of auditory cortical neurons, we demonstrate that subthreshold, NMDA-receptor-dependent spine calcium signals are abundant during up states, but almost absent during down states. In each neuron, about 500 nonclustered spines, which are widely dispersed throughout the dendritic field, are on average active during an up state. The same subset of spines is reliably active during both sensory stimulation and up states. Thus, spontaneously recurring up states evoke in these spines “patterned” calcium activity that may control consolidation of synaptic strength following epochs of sensory stimulation.