Sensing a binding event through charge transport variations using an aromatic oligoamide capsule
22-Jan-2021
Chem. Sci., 2021,12, 3743-3750, DOI: 10.1039/d0sc06060g
Chem. Sci.,, online article
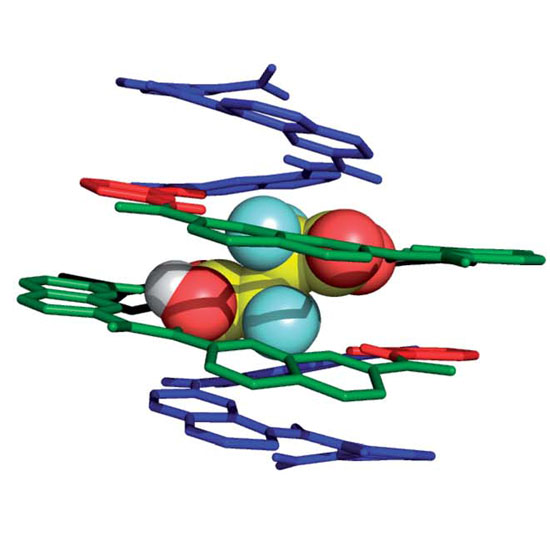
The selective binding properties of a 13-mer oligoamide foldamer capsule composed of 4 different aromatic subunits are reported. The capsule was designed to recognize dicarboxylic acids through multiple-point interactions owing to a combination of protonation/deprotonation events, H-bonding, and geometrical constraints imparted by the rigidity of the foldamer backbone. Compared to tartaric acid, binding of 2,2-difluorosuccinic acid or 2,2,3,3-tetrafluorosuccinic acid resulted in symmetry breaking due to deprotonation of only one of the two carboxylic acid groups of the encapsulated species as shown by NMR studies in solution and by single-crystal X-ray diffraction in the solid state. An analogous 14-mer foldamer capsule terminated with a thiol anchoring group was used to probe the complexation event in self-assembled monolayers on Au substrates. Ellipsometry and polarization-modulation infrared absorption-reflection spectroscopy studies were consistent with the formation of a single molecule layer of the foldamer capsule oriented vertically with respect to the surface. The latter underwent smooth complexation of 2,2-difluorosuccinic acid with deprotonation of one of the two carboxylic acid groups. A significant (80-fold) difference in the charge transport properties of the monolayer upon encapsulation of the dicarboxylic acid was evidenced from conducting-AFM measurements (S = 1.1 × 10−9vs. 1.4 × 10−11 ohm−1 for the empty and complexed capsule, respectively). The modulation in conductivity was assigned to protonation of the aromatic foldamer backbone.