- Home ·
- Publications ·
- Research Area F ·
- 2009 ·
Substrate Requirements for SPPL2b-dependent Regulated Intramembrane Proteolysis
27-Feb-2009
Lucas Martin, Regina Fluhrer, Christian Haass
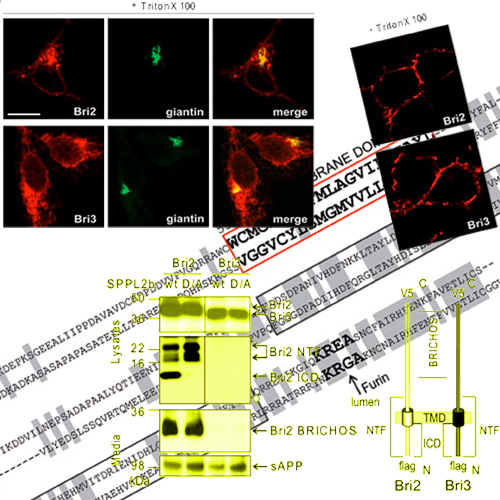
Intramembrane proteolysis is now widely recognized as an important physiological pathway required for reverse signaling and membrane protein degradation. Aspartyl intramembrane cleaving proteases of the GXGD-type play an important regulatory role in health and disease. Besides -secretase/presenilin, signal peptide peptidase (SPP) and SPP-like (SPPL) peptidases also belong to the family of GXGD-type aspartyl proteases. Although recently the first SPPL2a/b substrates have been identified, very little is known about substrate requirements, which allow them to be efficiently processed within the membrane. We demonstrate that similar to -secretase substrates, intramembrane proteolysis of Bri2 (Itm2b) is greatly facilitated by an initial shedding event mediated by ADAM-10. Serial deletions revealed that the length of the ectodomain negatively correlates with efficient intramembrane proteolysis. Bri3 (Itm2c), which is highly homologous to Bri2, fails to be shed. Failure of shedding of Bri3 is accompanied by a lack of intramembrane proteolysis by SPPL2b. Surprisingly, a low molecular weight membrane-retained stub of Bri3 also fails to be processed by SPPL2b, indicating that shedding per se is not sufficient for subsequent intramembrane proteolysis. Extensive domain swapping analysis reveals that primary sequence determinants within the intracellular domain and the transmembrane domain together with short luminal juxtamembrane sequences are required for efficient intramembrane proteolysis.