- Home ·
- Publications ·
- Research Area F ·
- 2009 ·
The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores
26-Jun-2009
Xiangang Zong, Michael Schieder, Hartmut Cuny, Stefanie Fenske, Christian Gruner, Katrin Rötzer, Oliver Griesbeck, Hartmann Harz, Martin Biel, Christian Wahl-Schott
European Journal of Physiology, 2009, DOI 10.1007/s00424-009-0690-y, published on 26.06.2009
European Journal of Physiology, online article
European Journal of Physiology, online article
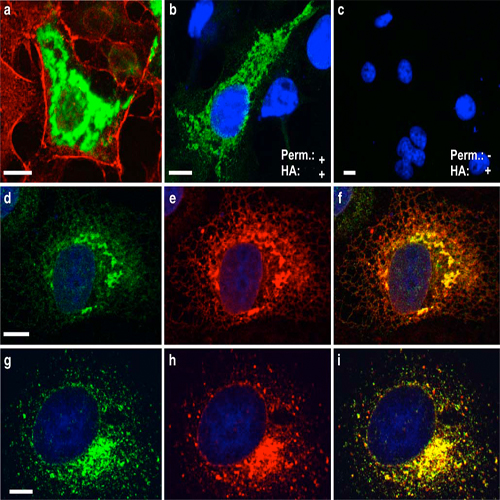
Second messenger-induced Ca2+-release from intracellular stores plays a key role in a multitude of physiological processes. In addition to 1,4,5-inositol trisphosphate (IP3), Ca2+, and cyclic ADP ribose (cADPR) that trigger Ca2+-release from the endoplasmatic reticulum (ER), nicotinic acid adenine dinucleotide phosphate (NAADP) has been identified as a cellular metabolite that mediates Ca2+-release from lysosomal stores. While NAADP-induced Ca2+-release has been found in many tissues and cell types, the molecular identity of the channel (s) conferring this release remained elusive so far. Here, we show that TPCN2, a novel member of the two-pore cation channel family, displays the basic properties of native NAADP-dependent Ca2+-release channels. TPCN2 transcripts are widely expressed in the body and encode a lysosomal protein forming homomers. TPCN2 mediates intracellular Ca2+-release after activation with lownanomolar concentrations of NAADP while it is desensitized by micromolar concentrations of this second messenger and is insensitive to the NAADP analog nicotinamide adenine dinucleotide phosphate (NADP). Furthermore, TPCN2- mediated Ca2+-release is almost completely abolished when the capacity of lysosomes for storing Ca2+ is pharmacologically blocked. By contrast, TPCN2-specific Ca2+-release is unaffected by emptying ER-based Ca2+ stores. In conclusion, these findings indicate that TPCN2 is a major component of the long-sought lysosomal NAADP-dependent Ca2+-release channel.