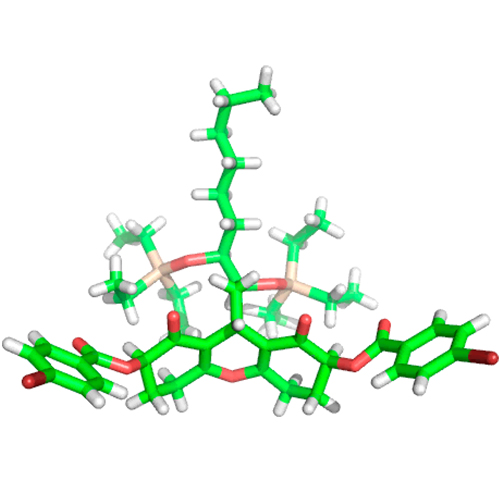
Total Synthesis of the Proposed Structure of Trichodermatide A
28-Aug-2014
J. Org. Chem., 2014, DOI: 10.1021/jo501206k, 79 (20), pp 9812–9817 published on 28.08.2014
A short total synthesis of the published structure of racemic trichodermatide A is reported. Our synthesis involves a Knoevenagel condensation/Michael addition sequence, followed by the formation of tricyclic hexahydroxanthene-dione and a diastereoselective bis-hydroxylation. The final product, the structure of which was confirmed by X-ray crystallography, has NMR spectra that are very similar, but not identical, to those of the isolated natural product. Quantum chemically computed 13C shifts agree well with the present NMR measurements.