A Conserved Proline Triplet in Val-tRNA Synthetase and the Origin of Elongation Factor P
09-Oct-2014
Cell Reports, 2014, http://dx.doi.org/10.1016/j.celrep.2014.09.008, 9, 1–8, published on 09.10.2014
Cell Reports, online artticle
Cell Reports, online artticle
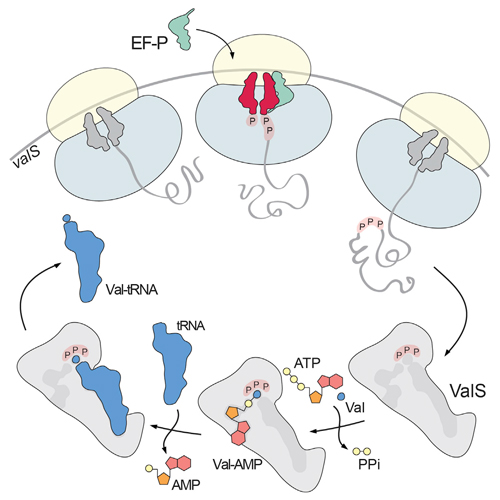
Bacterial ribosomes stall on polyproline stretches and require the elongation factor P (EF-P) to relieve the arrest. Yet it remains unclear why evolution has favored the development of EF-P rather than selecting against the occurrence of polyproline stretches in proteins. We have discovered that only a single polyproline stretch is invariant across all domains of life, namely a proline triplet in ValS, the tRNA synthetase, that charges tRNAVal with valine. Here, we show that expression of ValS in vivo and in vitro requires EF-P and demonstrate that the proline triplet located in the active site of ValS is important for efficient charging of tRNAVal with valine and preventing formation of mischarged Thr-tRNAVal as well as efficient growth of E. coli in vivo. We suggest that the critical role of the proline triplet for ValS activity may explain why bacterial cells coevolved the EF-P rescue system.