A Glycopeptide Dendrimer Inhibitor of the Galactose-Specific Lectin LecA and of Pseudomonas aeruginosa Biofilms
14-Sep-2011
Angewandte Chemie, 2011, DOI: 10.1002/anie.201104342, published on 17.04.2011
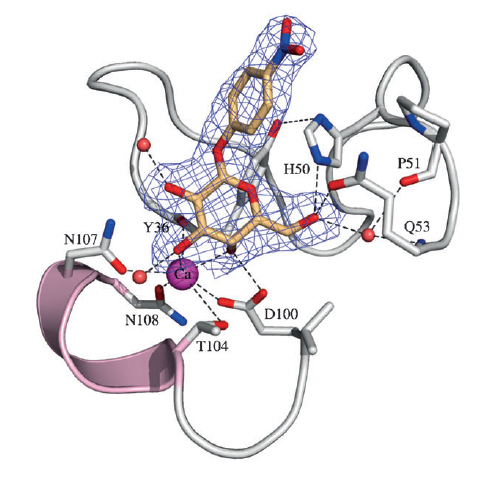
The spread of antibiotic resistant bacteria is one of the most pressing problems in human health today. In the case of the opportunistic pathogen Pseudomonas aeruginosa, which causes lethal airway infections in cystic fibrosis and immunocompromised patients, the formation of biofilms plays an important role in antibiotic resistance and disease progression. Biofilm formation is mediated in part by the galactosespecific lectin LecA (PA-IL) and the fucose-specific lectin LecB (PA-IIL) as evidenced by studies with deletion Mutants and the partial inhibitory effect of simple fucose and galactose derivatives in vitro and in vivo. Understanding the glycoconjugate–lectin interaction is a key feature in developing potent biofilm inhibitors. Capitalizing on the well-known cluster effect observed on binding of multivalent carbohydrates to lectins, we recently reported the first case of P. aeruginosa biofilm inhibition with a multivalent lectin inhibitor, the fucosylated glycopeptide dendrimer FD2 (cFuc-Lys-Pro-Leu) (Lys-Phe-Lys-Ile)2Lys-His-IleNH2, which targets LecB. Herein we report the first case of P. aeruginosa biofilm inhibition with a multivalent ligand targeting the galactose-specific lectin LecA, using the related beta-phenylgalactosyl peptide dendrimer GalAG2.