A tandem SH2 domain in transcription elongation factor Spt6 binds the phosphorylated RNA polymerase II CTD
06-Oct-2010
The Journal of Biological Chemistry, 2010, doi: 10.1074/jbc.M110.144568, 41597-41603 published on 06.10.2010
The Journal of Biological Chemistry, online article
The Journal of Biological Chemistry, online article
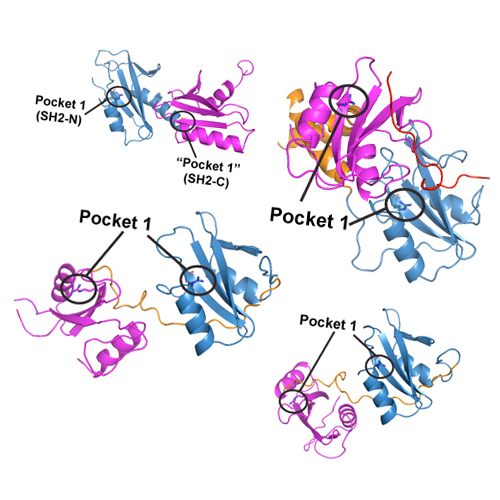
Spt6 is an essential transcription elongation factor and histone chaperone that binds the C-terminal repeat domain (CTD) of RNA polymerase (Pol) II. We show here that Spt6 contains a tandem SH2 domain with a novel structure and CTD-binding mode. The tandem SH2 domain binds to a serine 2-phosphorylated CTD peptide in vitro, whereas its N-terminal SH2 subdomain, which we previously characterized, does not. CTD binding requires a positively charged crevice in the C-terminal SH2 subdomain, which lacks the canonical phospho-binding pocket of SH2 domains and had previously escaped detection. The tandem SH2 domain is apparently required for transcription elongation in vivo, as its deletion in cells is lethal in the presence of 6-azauracil.