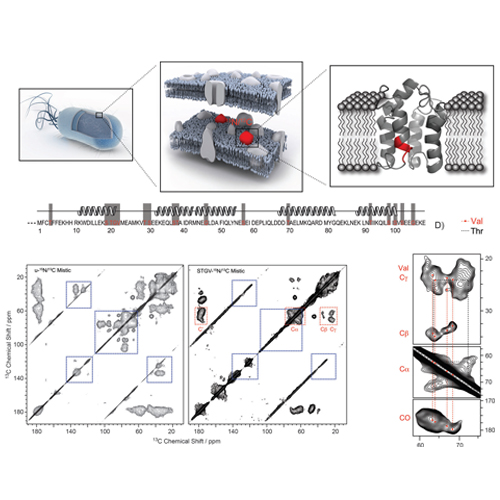
Characterization of Membrane Proteins in Isolated Native Cellular Membranes by Dynamic Nuclear Polarization Solid-State NMR Spectroscopy without Purification and Reconstitution
09-Jan-2012
Angewandte Chemie, 2012, DOI: 10.1002/anie.201104987, Volume 51, Issue 2, pages 432–435 published on 09.01.2012
Structural information is key for understanding biological processes. Insoluble proteins, like membrane proteins and amyloid fibrils, are a large class of proteins that are underrepresented in the protein data bank (PDB). As of today, only 7% of all entries in the PDB refer to either a membrane protein or an amyloid fibril structure (membrane protein: 4994 entries; amyloid fibril: 67 entries; total number of entries: 70,303; http://www.rcsb.org/pdb/home/home.do). Given the fact that many drugs target membrane proteins, involved in signal transduction,[1] structural information is highly desirable for a better understanding of the underlying biochemical mechanisms.