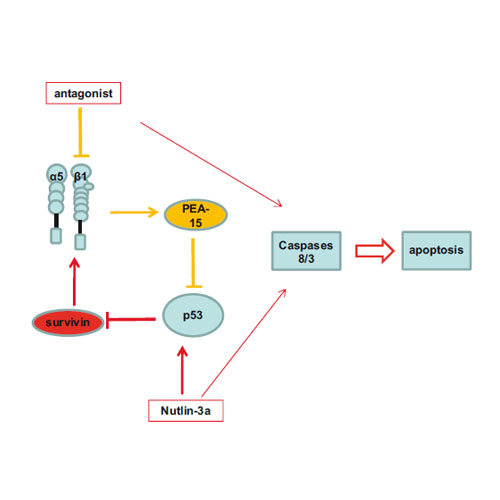
Integrin α5β1 and p53 convergent pathways in the control of anti-apoptotic proteins PEA-15 and survivin in high-grade glioma
16-Oct-2015
Cell Death & Differentiation, 23, 640-653, doi:10.1038/cdd.2015.131
Cell Death & Differentiation, online article
Integrin α5β1 expression is correlated with a worse prognosis in high-grade glioma. We previously unraveled a negative crosstalk between integrin α5β1 and p53 pathway, which was proposed to be part of the resistance of glioblastoma to chemotherapies. The restoration of p53 tumor-suppressor function is under intensive investigations for cancer therapy. However, p53-dependent apoptosis is not always achieved by p53-reactivating compounds such as Nutlin-3a, although full transcriptional activity of p53 could be obtained. Here we investigated whether integrin α5β1 functional inhibition or repression could sensitize glioma cells to Nutlin-3a-induced p53-dependent apoptosis. We discovered that α5β1 integrin-specific blocking antibodies or small RGD-like antagonists in association with Nutlin-3a triggered a caspase (Casp) 8/Casp 3-dependent strong apoptosis in glioma cells expressing a functional p53. We deciphered the molecular mechanisms involved and we showed the crucial role of two anti-apoptotic proteins, phosphoprotein enriched in astrocytes 15 (PEA-15) and survivin in glioma cell apoptotic outcome. PEA-15 is under α5β1 integrin/AKT (protein kinase B) control and survivin is a p53-repressed target. Moreover, interconnections between integrin and p53 pathways were revealed. Indeed PEA-15 repression by specific small-interfering RNA (siRNA)-activated p53 pathway to repress survivin and conversely survivin repression by specific siRNA decreased α5β1 integrin expression. This pro-apoptotic loop could be generalized to several glioma cell lines, whatever their p53 status, inasmuch PEA-15 and survivin protein levels were decreased. Our findings identify a novel mechanism whereby inhibition of α5β1 integrin and activation of p53 modulates two anti-apoptotic proteins crucially involved in the apoptotic answer of glioma cells. Importantly, our results suggest that high-grade glioma expressing high level of α5β1 integrin may benefit from associated therapies including integrin antagonists and repressors of survivin expression.