Modulation of the Hsp90 Chaperone Cycle by a Stringent Client Protein
20-Mar-2014
Molecular Cell, 2014, DOI: http://dx.doi.org/10.1016/j.molcel.2014.02.003, Volume 53, Issue 6, p941–953, published on 20.03.2014
Molecular Cell, online article
Molecular Cell, online article
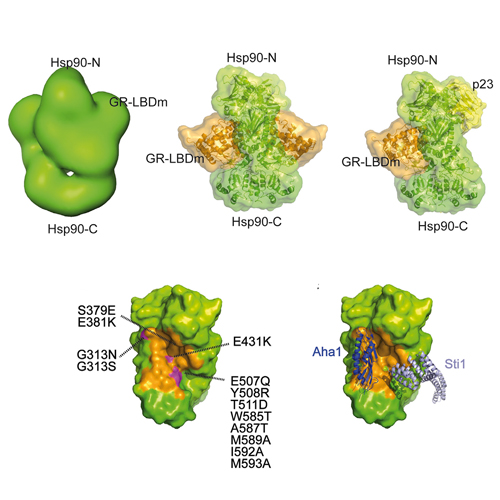
Hsp90 is the most abundant molecular chaperone in the eukaryotic cell. One of the most stringent clients is the glucocorticoid receptor (GR), whose in vivo function strictly depends on the interaction with the Hsp90 machinery. However, the molecular mechanism of this interaction has been elusive. Here we have reconstituted the interaction of Hsp90 with hormone-bound GR using purified components. Our biochemical and structural analyses define the binding site for GR on Hsp90 and reveal that binding of GR modulates the conformational cycle of Hsp90. FRET experiments demonstrate that a partially closed form of the Hsp90 dimer is the preferred conformation for interaction. Consistent with this, the conformational cycle of Hsp90 is decelerated, and its ATPase activity decreases. Hsp90 cochaperones differentially affect formation of the Hsp90-GR complex, serving as control elements for cycle progression and revealing an intricate interplay of client and cochaperones as molecular modulators of the Hsp90 machine.