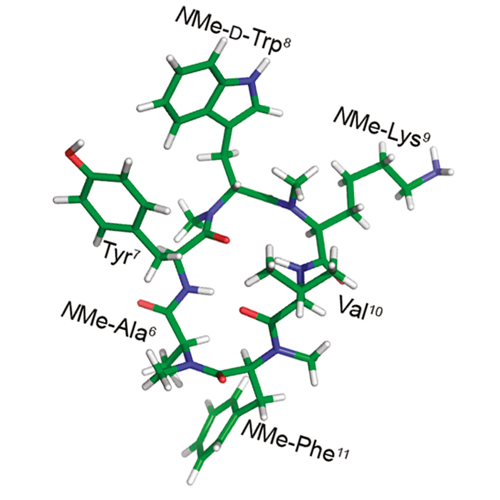
N-Methylated sst2 Selective Somatostatin Cyclic Peptide Analogue as a Potent Candidate for Treating Neurogenic Inflammation
04-Apr-2011
ACS Medicinal Chemistry Letters, 2011, DOI: 10.1021/ml200032v, published on 04.04.2011
A focused multiply N-methylated library of a cyclic hexapeptidic somatostatin analogue: MK678 cyclo(−MeAYwKVF−) was generated, which resulted in the unexpected observation of an efficacious tetra-N-methylated analogue, cyclo(−MeAYMewMeKVMeF−) with a potent inhibitory action on sensory neuropeptide release in vitro and on acute neurogenic inflammatory response in vivo. The analogue shows selectivity toward somatostatin receptor subtype 2 (sst2). Extensive 2D NMR spectroscopy and molecular dynamics simulation revealed the solution conformation of the analogue, which can be adopted as a lead for the further structure−activity relationship studies targeting neurogenic inflammation.