RNA polymerase II termination involves C-terminal-domain tyrosine dephosphorylation by CPF subunit Glc7
12-Jan-2014
Nature Structural & Molecular Biology, 2014, doi:10.1038/nsmb.2753, 21, 175–179 published on 12.01.2014
Nature Structural & Molecular Biology, online article
Nature Structural & Molecular Biology, online article
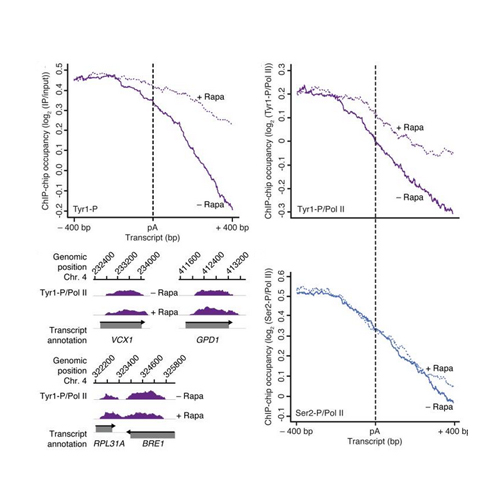
At the 3′ ends of protein-coding genes, RNA polymerase (Pol) II is dephosphorylated at tyrosine residues (Tyr1) of its C-terminal domain (CTD). In addition, the associated cleavage-and-polyadenylation factor (CPF) cleaves the transcript and adds a poly(a) tail. Whether these events are coordinated and how they lead to transcription termination remains poorly understood. Here we show that CPF from Saccharomyces cerevisiae is a Pol II–CTD phosphatase and that the CPF subunit Glc7 dephosphorylates Tyr1 in vitro. In vivo, the activity of Glc7 is required for normal Tyr1 dephosphorylation at the polyadenylation site, for recruitment of termination factors Pcf11 and Rtt103 and for normal Pol II termination. These results show that transcription termination involves Tyr1 dephosphorylation of the CTD and indicate that pre-mRNA processing by CPF and transcription termination are coupled via Glc7-dependent Pol II–Tyr1 dephosphorylation.