Structural Analysis of Large Protein Complexes Using Solvent Paramagnetic Relaxation Enhancements
25-Mar-2011
Angewandte Chemie, 2011, DOI: 10.1002/anie.201007168, Volume 50, Issue 17, pages 3993–3997 published on 25.03.2011
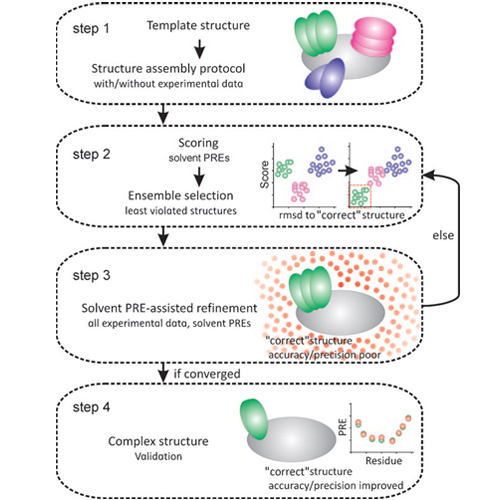
Understanding the function of biomolecular complexes requires their structural analysis at atomic resolution. To solve high-resolution structures by ab initio calculations typically data from NMR spectroscopy or X-ray crystallography are employed. In the latter approach, intrinsic flexibility and dynamics may prevent crystallization or introduce artificial conformations linked to crystal packing. Solution NMR spectroscopy does not suffer from such limitations, but is demanding because the adverse relaxation properties of large complexes may lead to extensive signal broadening and severe spectral overlap. Consequently, only sparse restraints can be obtained from such complexes by NMR experiments. Provided that the structures of the individual components of the complex (i.e. proteins, DNA/RNA) are available and that no large-scale conformational changes occur upon complex formation, experimental and computational approaches can be used to obtain the quaternary arrangement of complexes.