Structural Properties of EGCG-Induced, Nontoxic Alzheimer's Disease Aβ Oligomers
24-Aug-2012
Journal of Molecular Biology, 2012, doi:10.1016/j.jmb.2012.01.013, Volume 421, Issues 4–5, Pages 517–524 published on 24.08.2012
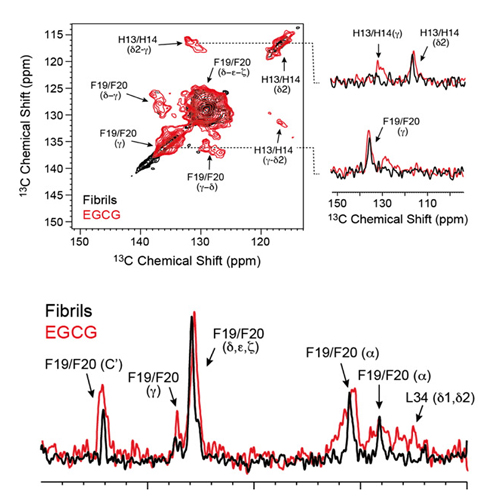
The green tea compound epigallocatechin-3-gallate (EGCG) inhibits Alzheimer's disease β-amyloid peptide (Aβ) neurotoxicity. Solution-state NMR allows probing initial EGCG–Aβ interactions. We show that EGCG-induced Aβ oligomers adopt a well-defined structure and are amenable for magic angle spinning solid-state NMR investigations. We find that EGCG interferes with the aromatic hydrophobic core of Aβ. The C-terminal part of the Aβ peptide (residues 22–39) adopts a β-sheet conformation, whereas the N-terminus (residues 1–20) is unstructured. The characteristic salt bridge involving residues D23 and K28 is present in the structure of these oligomeric Aβ aggregates as well. The structural analysis of small-molecule-induced amyloid aggregates will open new perspectives for Alzheimer's disease drug development.