Structure, phosphorylation and U2AF65 binding of the N-terminal domain of splicing factor 1 during 30-splice site recognition
21-Nov-2012
Nucleic Acids Research, 2012, doi:10.1093/nar/gks1097, Vol. 41, No. 2 ,1343–1354 published on 21.11.2012
Nucleic Acids Research, online article
Nucleic Acids Research, online article
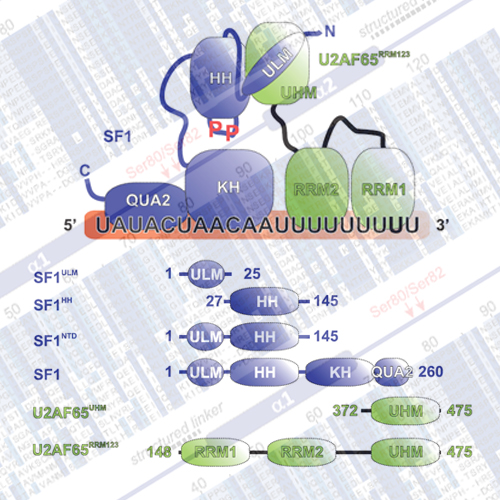
Recognition of the 30-splice site is a key step in premRNA splicing and accomplished by a dynamic complex comprising splicing factor 1 (SF1) and the U2 snRNP auxiliary factor 65-kDa subunit (U2AF65). Both proteins mediate protein–protein and protein–RNA interactions for cooperative RNA-binding during spliceosome assembly. Here, we report the solution structure of a novel helix-hairpin domain in the N-terminal region of SF1 (SF1NTD). The nuclear magnetic resonance- and small-angle X-ray scattering-derived structure of a complex of the SF1NTD with the C-terminal U2AF homology motif domain of U2AF65 (U2AF65UHM) reveals that, in addition to the known U2AF65UHM–SF1 interaction, the helix-hairpin domain forms a secondary, hydrophobic interface with U2AF65UHM, which locks the orientation of the two subunits. Mutational analysis shows that the helix hairpin is essential for cooperative formation of the ternary SF1–U2AF65–RNA complex. We further show that tandem serine phosphorylation of a conserved Ser80-Pro81-Ser82- Pro83 motif rigidifies a long unstructured linker in the SF1 helix hairpin. Phosphorylation does not significantly alter the overall conformations of SF1, SF1–U2AF65 or the SF1–U2AF65–RNA complexes, but slightly enhances RNA complexes, but slightly enhances RNA binding. Our results indicate that the helix-hairpin domain of SF1 is required for cooperative 30-splice site recognition presumably by stabilizing a unique quaternary arrangement of the SF1–U2AF65–RNA complex.