The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP
16-Dec-2007
Molecular Microbiology, 2007, 67 (3), 570-83 published on 16.12.2007
Molecular Microbiology, online article
Molecular Microbiology, online article
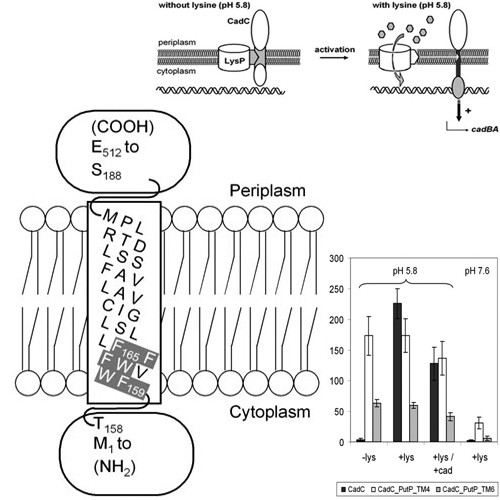
In an acidic (pH 5.8) and lysine-rich environment Escherichia coli induces expression of the cadBA operon which encodes CadA, catalysing the decarboxylation of lysine to cadaverine, and CadB, the lysine/cadaverine antiporter. cadBA expression is dependent on CadC, a membrane-integrated transcriptional activator which belongs to the ToxR-like protein family and directly binds to the DNA in the cadBA promoter region. Here we describe that CadC senses the extracellular lysine not directly but indirectly requiring the interplay with the lysine permease LysP. Biochemical analyses of isolated CadC or the periplasmic domain of CadC (CadC188–512) revealed an unexpectedly low affinity for lysine, making it unlikely that CadC is a direct sensor for this substrate. Moreover, CadC hybrid proteins, in which the transmembrane domain or single amino acids were replaced, supported lysine-independent cadBA expression but retained a pH-dependent regulation. These CadC mutants were resistant to the effect of an overproduction of LysP, which represses cadBA expression in wild-type cells. Our results suggest a model according to which CadC is inactivated by an interaction with LysP at a low external lysine concentration. When lysine is abundantly available, the interaction between LysP and CadC is released, and CadC becomes susceptible to activation by low pH.