The RGD motif in fi bronectin is essential for development but dispensable for fibril assembly
02-Jul-2007
The Journal of Cell Biology, 2007, 178 (1), 167-78 published on 02.07.2007
Journal of Cell Biology, online article
Journal of Cell Biology, online article
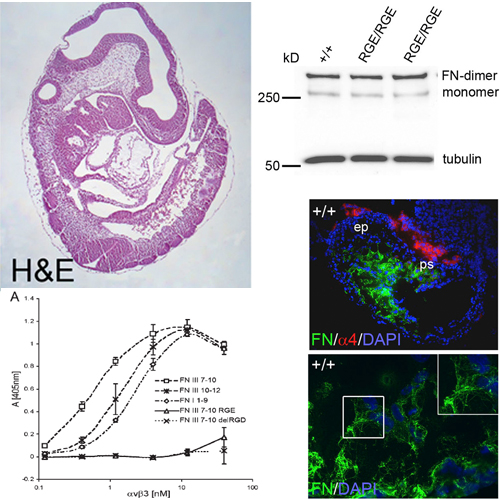
Fibronectin (FN) is secreted as a disulfi de-bonded FN dimer. Each subunit contains three types of repeating modules: FN-I, FN-II, and FN-III. The interactions of α5β1 or αv integrins with the RGD motif of FN-III repeat 10 (FN-III10) are considered an essential step in the assembly of FN fi brils. To test this hypothesis in vivo, we replaced the RGD motif with the inactive RGE in mice. FN-RGE homozygous embryos die at embryonic day 10 with shortened posterior trunk, absent tail bud–derived somites, and severe vascular defects resembling the phenotype of α5 integrin–defi cient mice. Surprisingly, the absence of a functional RGD motif in FN did not compromise assembly of an FN matrix in mutant embryos or on mutant cells. Matrix assembly assays and solid-phase binding assays reveal that αvβ3 integrin assembles FNRGE by binding an isoDGR motif in FN-I5, which is generated by the nonenzymatic rearrangement of asparagines (N) into an iso-aspartate (iso-D). Our fi ndings demonstrate that FN contains a novel motif for integrin binding and fi bril formation whose activity is controlled by amino acid modifi cation.