Ultra-high resolution in MAS solid-state NMR of perdeuterated proteins: Implications for structure and dynamics
05-Jan-2012
Journal of Magnetic Resonance, 2012, http://dx.doi.org/10.1016/j.jmr.2011.12.017, Volume 216,Pages 1–12 published on 05.01.2012
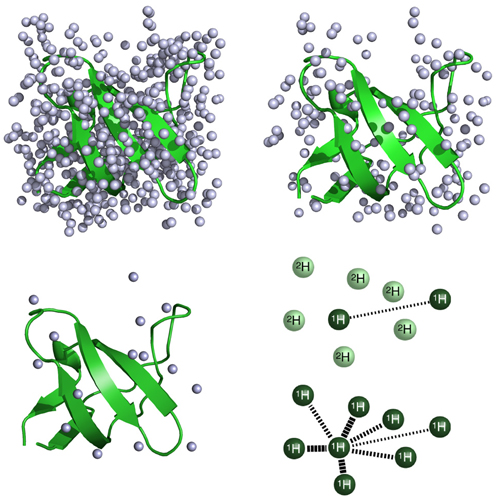
High resolution proton spectra are obtained in MAS solid-state NMR in case samples are prepared using perdeuterated protein and D2O in the recrystallization buffer. Deuteration reduces drastically 1H, 1H dipolar interactions and allows to obtain amide proton line widths on the order of 20 Hz. Similarly, high-resolution proton spectra of aliphatic groups can be obtained if specifically labeled precursors for biosynthesis of methyl containing side chains are used, or if limited amounts of H2O in the bacterial growth medium is employed. This review summarizes recent spectroscopic developments to access structure and dynamics of biomacromolecules in the solid-state, and shows a number of applications to amyloid fibrils and membrane proteins.