Unveiling the Long-Held Secrets of the 26S Proteasome
03-Sep-2013
Structure, 2013, DOI: 10.1016/j.str.2013.08.010, Volume 21, Issue 9, 1551-1562, published on 03.09.2013
Structure, online article
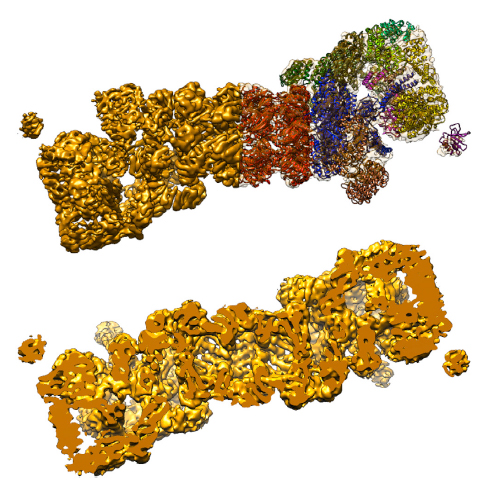
The 26S proteasome is a 2.5 MDa molecular machine for the degradation of substrates of the ubiquitin-proteasome pathway with a key role in cellular proteostasis. Until recently, only the structure of its core particle, the 20S proteasome, could be studied in detail, whereas the 19S regulatory particle or the holocomplex remained elusive. Novel integrative approaches have now revealed the molecular architecture of the entire complex and provided the first insights into the conformational changes during its functional cycle. Here we review the problems in structural studies of the 26S proteasome, the methods that made possible its structure determination, the architectural principles of the holocomplex, and its conformational space. These advances provide valuable insights into the mechanism of substrate recruitment and processing preceding their destruction in the 20S core particle.