Arginine Citrullination at the C-Terminal Domain Controls RNA Polymerase II Transcription
21-Nov-2018
Molecular Cell, 73, 1–13, https://doi.org/10.1016/j.molcel.2018.10.016
Molecular Cell, online article
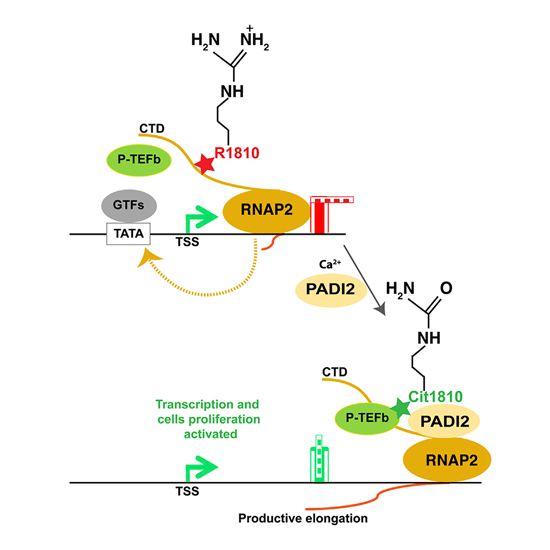
The post-translational modification of key residues at the C-terminal domain of RNA polymerase II (RNAP2-CTD) coordinates transcription, splicing, and RNA processing by modulating its capacity to act as a landing platform for a variety of protein complexes. Here, we identify a new modification at the CTD, the deimination of arginine and its conversion to citrulline by peptidyl arginine deiminase 2 (PADI2), an enzyme that has been associated with several diseases, including cancer. We show that, among PADI family members, only PADI2 citrullinates R1810 (Cit1810) at repeat 31 of the CTD. Depletion of PADI2 or loss of R1810 results in accumulation of RNAP2 at transcription start sites, reduced gene expression, and inhibition of cell proliferation. Cit1810 is needed for interaction with the P-TEFb (positive transcription elongation factor b) kinase complex and for its recruitment to chromatin. In this way, CTD-Cit1810 favors RNAP2 pause release and efficient transcription in breast cancer cells.