Synthesis and Macrodomain Binding of Mono-ADP-Ribosylated Peptides
26-Aug-2016
Angew. Chem. Int. Ed., Volume 55, Issue 36, Pages 10634–10638, DOI: 10.1002/anie.201604058
Angew. Chem. Int. Ed., online article
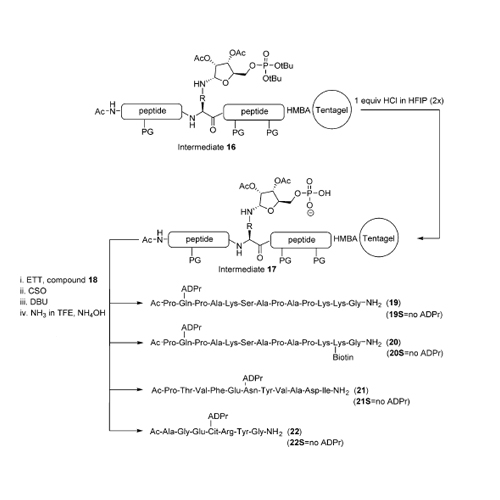
Mono-ADP-ribosylation is a dynamic posttranslational modification (PTM) with important roles in signaling. Mammalian proteins that recognize or hydrolyze mono-ADP-ribosylated proteins have been described. We report the synthesis of ADP-ribosylated peptides from the proteins histone H2B, RhoA and, HNP-1. An innovative procedure was applied that makes use of pre-phosphorylated amino acid building blocks. Binding assays revealed that the macrodomains of human MacroD2 and TARG1 exhibit distinct specificities for the different ADP-ribosylated peptides, thus showing that the sequence surrounding ADP-ribosylated residues affects the substrate selectivity of macrodomains.