The ATPase domain of ISWI is an autonomous nucleosome remodeling machine
02-Dec-2012
Nature Structural & Molecular Biology, 2012, doi:10.1038/nsmb.2457, 20, 82–89 (2013), published on 02.12.2012
Nature Structural & Molecular Biology, online article
Nature Structural & Molecular Biology, online article
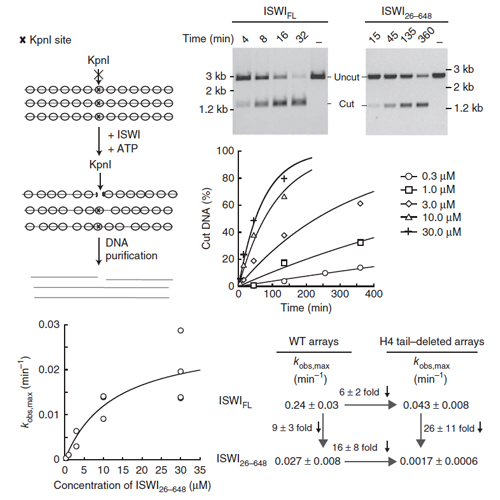
ISWI slides nucleosomes along DNA, enabling the structural changes of chromatin required for the regulated use of eukaryotic genomes. Prominent mechanistic models imply cooperation of the ISWI ATPase domain with a C-terminal DNA-binding function residing in the HAND-SANT-SLIDE (HSS) domain. Contrary to these models, we show by quantitative biochemical means that all fundamental aspects of nucleosome remodeling are contained within the compact ATPase module of Drosophila ISWI. This domain can independently associate with DNA and nucleosomes, which in turn activate ATP turnover by inducing a conformational change in the enzyme, and it can autonomously reposition nucleosomes. The role of the HSS domain is to increase the affinity and specificity for nucleosomes. Nucleosome-remodeling enzymes may thus have evolved directly from ancestral helicase-type motors, and peripheral domains have furnished regulatory capabilities that bias the remodeling reaction toward different structural outcomes.