A Dynamic Equilibrium of Three Hydrogen-Bond Conformers Explains the NMR Spectrum of the Active Site of Photoactive Yellow Protein
14-Sep-2016
J. Chem. Theory Comput., 12 (10), pp 5170–5178, DOI: 10.1021/acs.jctc.6b00359
J. Chem. Theory Comput., online article
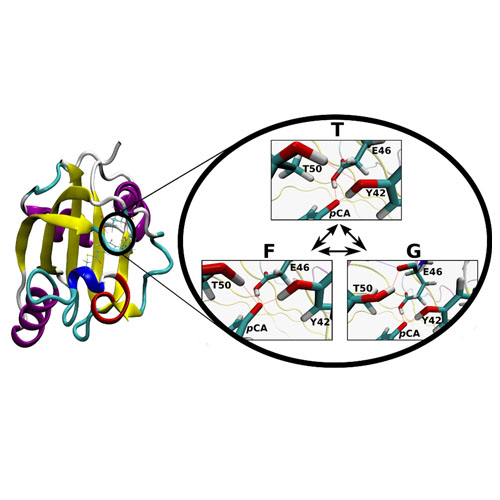
A theoretical study on the NMR shifts of the hydrogen bond network around the chromophore, para-coumaric acid (pCA), of photoactive yellow protein (PYP) is presented. Previous discrepancies between theoretical and experimental studies are resolved by our findings of a previously unknown rapid conformational exchange near the active site of PYP. This exchange caused by the rotation of Thr50 takes place in the ground state of PYP’s active site and results in three effectively energetically equal conformations characterized by the formation of new hydrogen bonds, all of which contribute to the overall NMR signals of the investigated protons. In light of these findings, we are able to successfully explain the experimental results and provide valuable insight into the behavior of PYP in solution. We further investigated related PYP mutants (T50V, E46Q, and Y42F), and found the same conformational exchange in E46Q and Y42F to be responsible for the experimentally observed NMR and UV/vis spectra.