Fast-folding α-helices as reversible strain absorbers in the muscle protein myomesin
08-Aug-2011
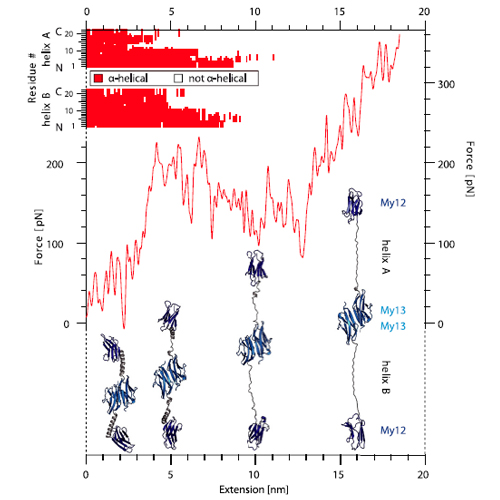
The highly oriented filamentous protein network of muscle constantly experiences significant mechanical load during muscle operation. The dimeric protein myomesin has been identified as an important M-band component supporting the mechanical integrity of the entire sarcomere. Recent structural studies have revealed a long αlpha-helical linker between the C-terminal immunoglobulin (Ig) domains My12 and My13 of myomesin. In this paper, we have used single-molecule force spectroscopy in combination with molecular dynamics simulations to characterize the mechanics of the myomesin dimer comprising immunoglobulin domains My12–My13. We find that at forces of approximately 30 pN the αlpha-helical linker reversibly elongates allowing the molecule to extend by more than the folded extension of a full domain. Highresolution measurements directly reveal the equilibrium folding/ unfolding kinetics of the individual helix. We show that αlpha-helix unfolding mechanically protects the molecule homodimerization from dissociation at physiologically relevant forces. As fast and reversible molecular springs the myomesin αlpha-helical linkers are an essential component for the structural integrity of the M band.