Folding and Unfolding of Light-Triggered beta-Hairpin Model Peptides
03-Jan-2011
J. Phys. Chem. B, 2011, DOI: 10.1021/jp107683d, 115 (18), pp 5219–5226 published on 03.01.2011
J. Phys. Chem. B, online article
J. Phys. Chem. B, online article
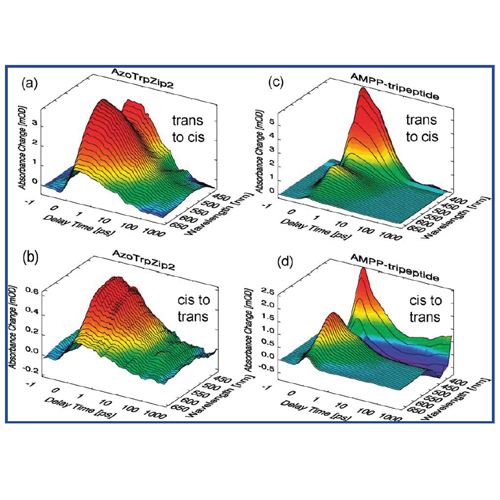
Ultrafast spectroscopy in the visible and midinfrared is used to study the reaction dynamics of two lighttriggered model peptides containing an azobenzene derivative as a switching element. One model peptide, the AzoTrp- Zip2, forms a beta-hairpin structure in the cis form of the chromophore. This peptide is compared to the core structure consisting of the chromophore and the two flanking amino acid residues, used as a minimal model. This combination of experiments performed in different spectral ranges on peptides of different sizes allows for improved insight into light triggered reaction dynamics. The kinetics observed for the core structure are directly connected to the switching process of the chromophore and are finished on the 10 ps time scale.The trans-to-cis reaction of AzoTrpZip2, leading to the formation of the β-hairpin structure involves ultrafast processes on the 100 ps time scale, which are directly related to the relaxation of the strain between the isomerized molecular switch and the two peptide strands. IR-signatures characteristic for changes in interstrand interactions are absent on the <1 ns time scale. Thus folding into the beta-hairpin structure occurs on a much longer time scale. In the cis-to-trans unfolding reaction, all IR signatures related to changes in interstrand interactions occur within 1 ns, in a time range where visible spectroscopy reveals the final decay of the intramolecular strain. Apparently unfolding of AzoTrpZip2 is to a large extent a fast, driven process.