Hydrophobic and Hofmeister Effects on the Adhesion of Spider Silk Proteins onto Solid Substrates: An AFM-Based Single-Molecule Study
19-Feb-2008
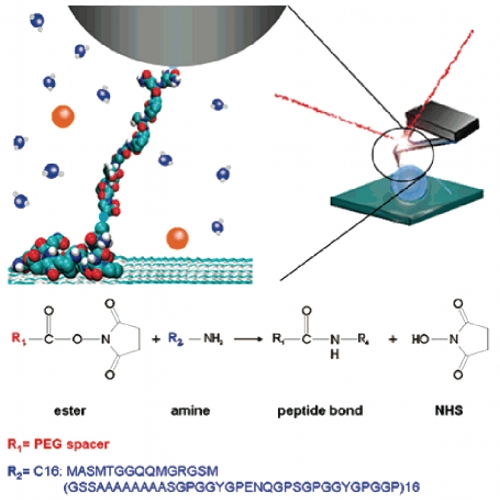
AFM-based single-molecule force spectroscopy has been used to study the effect of Hofmeister salts and protein hydrophobicity on the adhesion of recombinant spider silk proteins onto solid substrates. Therefore, a molecular probe consisting of a spider silk protein and an AFM tip has been developed, which (i) is a well-defined, small system that can be simulated by molecular dynamics simulations, (ii) allows access to the whole soluble concentration range for ions, and (iii) provides the distribution of desorption forces rather than just ensemble-averaged mean values. The measured desorption forces follow the Hofmeister series for anions (H2PO4-, Cl-, I-) with a stabilizing energy of more than 15 kBT for 5 M NaH2PO4. Moreover, this effect is influenced by the hydrophobicity of the spider silk protein, indicating that hydrophobic and Hofmeister effects are closely related.