Knotting and unknotting of a protein in single molecule experiments
05-Jul-2016
PNAS, vol. 113, no. 27, 7533–7538, doi: 10.1073/pnas.1600614113
PNAS, online article
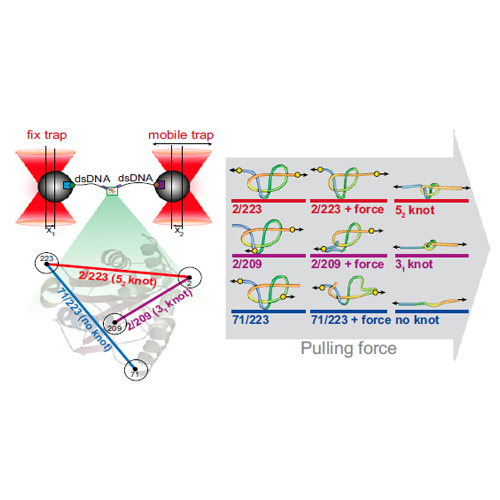
Spontaneous folding of a polypeptide chain into a knotted structure remains one of the most puzzling and fascinating features of protein folding. The folding of knotted proteins is on the timescale of minutes and thus hard to reproduce with atomistic simulations that have been able to reproduce features of ultrafast folding in great detail. Furthermore, it is generally not possible to control the topology of the unfolded state. Single-molecule force spectroscopy is an ideal tool for overcoming this problem: by variation of pulling directions, we controlled the knotting topology of the unfolded state of the 52-knotted protein ubiquitin C-terminal hydrolase isoenzyme L1 (UCH-L1) and have therefore been able to quantify the influence of knotting on its folding rate. Here, we provide direct evidence that a threading event associated with formation of either a 31 or 52 knot, or a step closely associated with it, significantly slows down the folding of UCH-L1. The results of the optical tweezers experiments highlight the complex nature of the folding pathway, many additional intermediate structures being detected that cannot be resolved by intrinsic fluorescence. Mechanical stretching of knotted proteins is also of importance for understanding the possible implications of knots in proteins for cellular degradation. Compared with a simple 31 knot, we measure a significantly larger size for the 52 knot in the unfolded state that can be further tightened with higher forces. Our results highlight the potential difficulties in degrading a 52 knot compared with a 31 knot.