Microdomain Formation Controls Spatiotemporal Dynamics of Cell-Surface Glycoproteins
20-Aug-2015
ChemBioChem, 2015, Volume 16, Issue 14, pages 2023–2028, DOI: 10.1002/cbic.201500361
ChemBioChem, online article
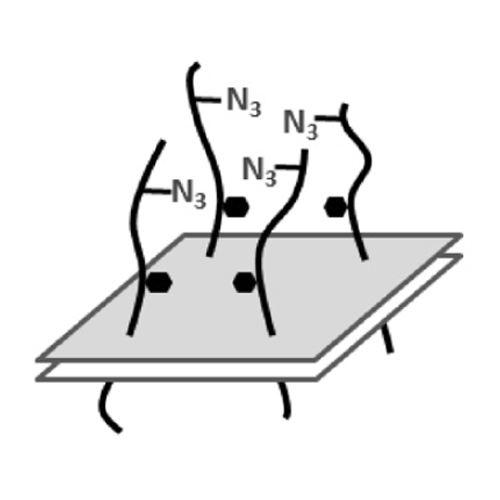
The effect of galectin-mediated microdomain formation on the spatiotemporal dynamics of glycosylated membrane proteins in human microvascular endothelial cells (HMEC-1) was studied qualitatively and quantitatively by high-resolution fluorescence microscopy and artificially mimicked by metabolic glycoprotein engineering. Two types of membrane proteins, sialic acid-bearing proteins (SABPs) and mucin-type proteins (MTPs), were investigated. For visualization they were metabolically labeled with azido sugars and then coupled to a cyclooctyne-conjugated fluorescent dye by click chemistry. Both spatial (diffusion) and temporal (residence time) dynamics of SABPs and MTPs on the membrane were investigated after treatment with exogenous galectin-1 or -3. Strong effects of galectin-mediated lattice formation were observed for MTPs (decreased spatial mobility), but not for SABPs. Lattice formation also strongly decreased the turnover of MTPs (increased residence time on the cell membrane). The effects of galectin-mediated crosslinking was accurately mimicked by streptavidin-mediated crosslinking of biotin-tagged glycoproteins and verified by single-molecule tracking. This technique allows the induction of crosslinking of membrane proteins under precisely controlled conditions, thereby influencing membrane residence time and the spatial dynamics of glycans on the cell membrane in a controlled way.