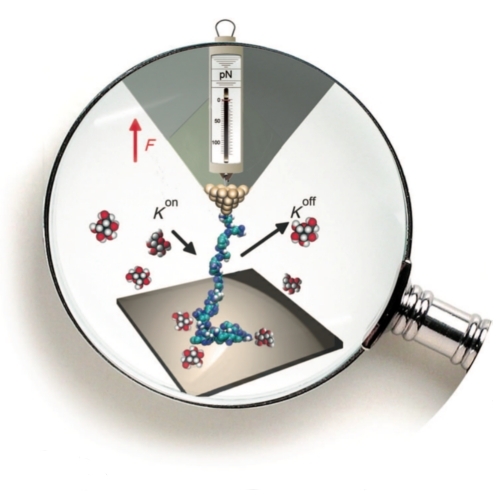
Pulling a Single Polymer Molecule off a Substrate Reveals the Binding Thermodynamics of Cosolutes
28-Jun-2010
Angewandte Chemie Int. Ed., 2010, doi:10.1002/anie.200907098, published on 28.06.2010
Angewandte Chemie Int. Ed., online article
Angewandte Chemie Int. Ed., online article
Cosolutes, such as ions, ligands, or small biomolecules, can cause a protein to fold into its biologically functional native form, to associate, to adhere or desorb from an interface, or even change its mechanical properties.[1–5] At the same time, the interaction of cosolutes with macromolecules, such as proteins, lipids and DNA, are themselves modified by changes in the environment, in particular by binding to surfaces, for example the cell membrane or the surface of histones and microtubules.[6,7] We combine single molecule atomic force microscopy with thermodynamic modeling and thereby extract the binding parameters of cosolutes onto a macro- molecule in solution as well as in its surface adsorbed state. Specifically, we obtain values for the adsorption site lengths (inverse maximal line densities) and the association constants. The considerable effect of substrates on cosolute binding is demonstrated with glucose as cosolute, poly(allylamine) as polymer, and surgical stainless steel or oxidized diamond as substrates.