Testing the diffusing boundary model for the helix–coil transition in peptides
06-Aug-2013
PNAS, 2013, doi: 10.1073/pnas.1303515110, vol. 110 no. 32 12905-12910 published on 06.08.2013
PNAS, online article
PNAS, online article
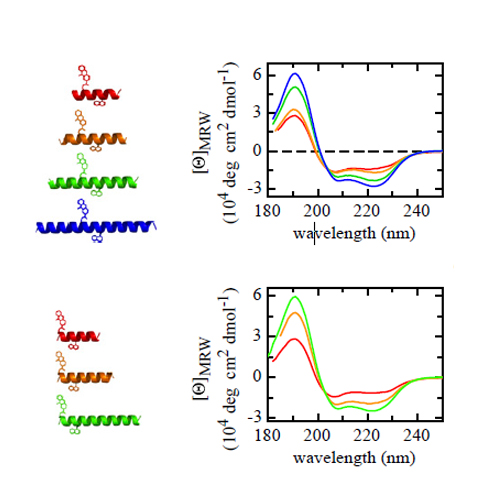
The dynamics of peptide α-helices have been studied extensively for many years, and the kinetic mechanism of the helix–coil dynamics has been discussed controversially. Recent experimental results have suggested that equilibrium helix–coil dynamics are governed by movement of the helix/coil boundary along the peptide chain, which leads to slower unfolding kinetics in the helix center compared with the helix ends and position-independent helix formation kinetics. We tested this diffusion of boundary model in helical peptides of different lengths by triplet-triplet energy transfer measurements and compared the data with simulations based on a kinetic linear Ising model. The results show that boundary diffusion in helical peptides can be described by a classical, Einstein-type, 1D diffusion process with a diffusion coefficient of 2.7⋅107 (amino acids)2/s or 6.1⋅10−9 cm2/s. In helices with a length longer than about 40 aa, helix unfolding by coil nucleation in a helical region occurs frequently in addition to boundary diffusion. Boundary diffusion is slowed down by helix-stabilizing capping motifs at the helix ends in agreement with predictions from the kinetic linear Ising model. We further tested local and nonlocal effects of amino acid replacements on helix–coil dynamics. Single amino acid replacements locally affect folding and unfolding dynamics with a ϕf-value of 0.35, which shows that interactions leading to different helix propensities for different amino acids are already partially present in the transition state for helix formation. Nonlocal effects of amino acid replacements only influence helix unfolding (ϕf = 0) in agreement with a diffusing boundary mechanism.