Time-resolved infrared studies of the unfolding of a light triggered b-hairpin peptide
06-Feb-2018
Chemical Physics, 512 (2018) 116–121, https://doi.org/10.1016/j.chemphys.2018.02.003
Chemical Physics, online article
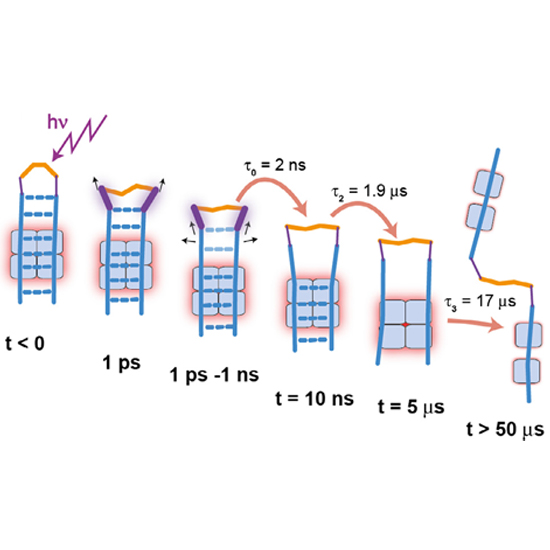
The light triggered unfolding reaction of the azobenzene peptide AzoTrpZip2 is investigated from 1 ps to 100 µs. Absorption changes show that the unfolding is a multistep process with the initial breaking of the hydrogen bonds in the vicinity of the AMPP chromophore on the 1 ns time scale followed by the disappearance of the remaining interstrand hydrogen bonds of the native hairpin structure with a 1.9 µs process. Subsequently, the hydrophobic core structure still stabilising a hairpin-like pattern rearranges in a 17 µs process. The strong slowing down of this reaction at lower temperature points to a barrier height in the range of 60 kJ/mol.