Unraveling the Base Excision Repair Mechanism of Human DNA Glycosylase
30-Jul-2015
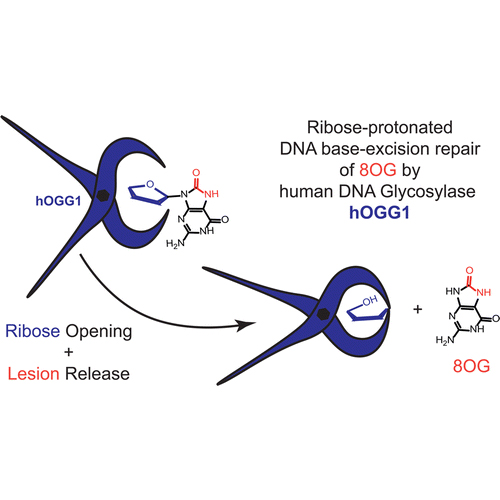
Human DNA glycosylase, hOGG1, is known to perform DNA repair by cleaving oxidized guanine (8OG) from the DNA. Despite numerous experimental and theoretical investigations, the underlying selective molecular mechanism has remained a mystery. Here we present a mechanism that explains how hOGG1’s catalytic pocket is able to host an undamaged guanine base, but is not able to cleave it from the DNA. Using linear-scaling quantum mechanics/molecular mechanics (QM/MM) techniques with more than 500 atoms in the QM-region, we have investigated previously proposed mechanisms that all rely on protonating the 8OG nucleobase. We have found that the repair mechanisms propagated in the literature to this date are not capable of differentiating between the G and 8OG nucleobase. Besides this nonselectivity, they also involve reaction barriers that are too high, hence rendering the corresponding reaction intermediates inaccessible. Instead, we present a completely different reaction mechanism, where hOGG1 initially targets the ribose moiety of the substrate and cleaves the glycosidic bond at the very last stage. Our ribose-protonated repair mechanism is not only energetically more preferable, but also explains the selectivity utilized by hOGG1 to block processing a guanine base.