α-Keto Phenylamides as P1′-Extended Proteasome Inhibitors
01-Aug-2014
ChemMedChem, 2014, DOI: 10.1002/cmdc.201402244, Volume 9, Issue 11, pages 2557–2564, published on 01.08.2014
ChemMedChem, online article
ChemMedChem, online article
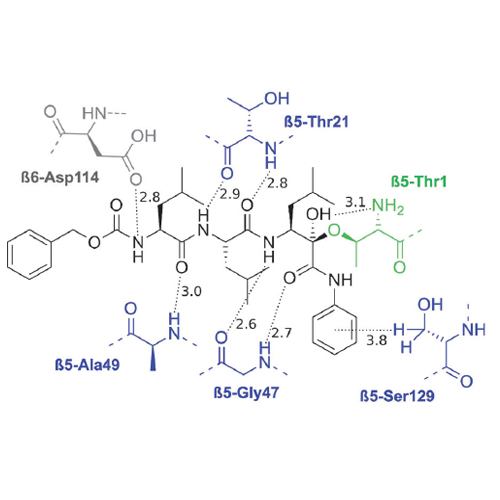
The major challenge for proteasome inhibitor design lies in achieving high selectivity for, and activity against, the target, which requires specific interactions with the active site. Novel ligands aim to overcome off-target-related side effects such as peripheral neuropathy, which is frequently observed in cancer patients treated with the FDA-approved proteasome inhibitors bortezomib or carfilzomib. A systematic comparison of electrophilic headgroups recently identified the class of α-keto amides as promising for next generation drug development. On the basis of crystallographic knowledge, we were able to develop a structure–activity relationship (SAR)-based approach for rational ligand design using an electronic parameter (Hammett’s σ) and in silico molecular modeling. This resulted in the tripeptidic α-keto phenylamide BSc4999 [(S)-3-(benzyloxycarbonyl-(S)-leucyl-(S)-leucylamino)-5-methyl-2-oxo-N-(2,4-dimethylphenyl)hexanamide, 6 a], a highly potent (IC50=38 nM), cell-permeable, and slowly reversible covalent inhibitor which targets both the primed and non-primed sites of the proteasome’s substrate binding channel as a special criterion for selectivity. The improved inhibition potency and selectivity of this new α-keto phenylamide makes it a promising candidate for targeting a wider range of tumor subtypes than commercially available proteasome inhibitors and presents a new candidate for future studies.