Phosphomimicking within the transit peptide of pHCF136 leads to reduced photosystem II accumulation in vivo
29-Apr-2015
FEBS Letters, Volume 589, Issue 12, Pages 1301–1307, http://dx.doi.org/10.1016/j.febslet.2015.04.033
FEBS Letters, online article
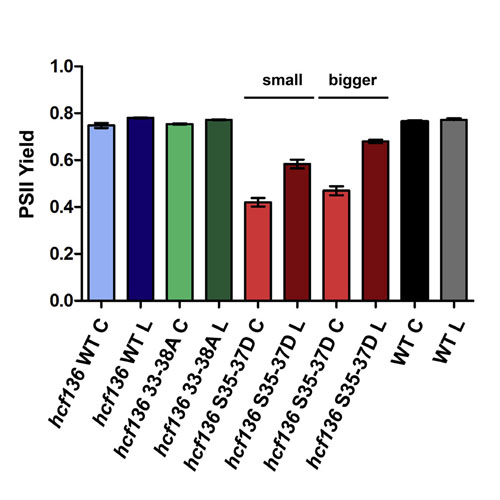
Most chloroplast resident proteins are equipped with N-terminal transit peptides to ensure targeting from the cytosol to the organelle. Import rates can be modulated by phosphorylation and 14-3-3 binding within the transit peptides. Using the phosphorylatable preprotein pHCF136, a photosystem II assembly factor, we investigated the function of preprotein phosphorylation in vivo by complementing the seedling lethal hcf136 mutant. HCF136 constructs containing mutations within the 14-3-3 binding site were generated, either abolishing or mimicking phosphorylation. Interestingly, phosphomimicking reduced the import rate and the hcf136 phenotype could only be partially rescued, as shown by hampered photosystem II complex accumulation, which was most prominently observed in cotyledons.