The charged linker region is an important regulator of Hsp90 function
09-Oct-2009
J. Biol. Chem., 2009, 284(34), 22559–22567, doi:10.1074/jbc.M109.031658 published on 09.10.2009
J. Biol. Chem., online article
J. Biol. Chem., online article
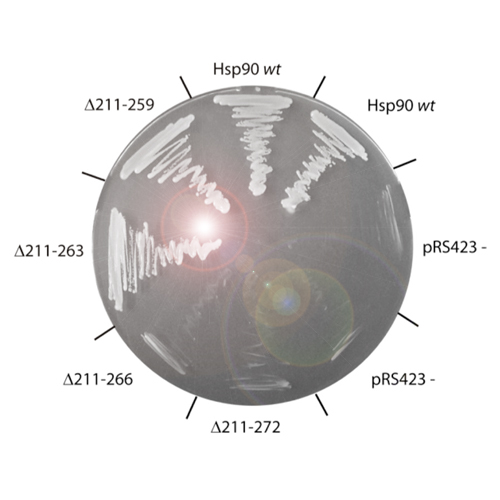
Hsp90 is an ATP-dependent molecular chaperone which assists the maturation of a large set of target proteins. Members of the highly conserved Hsp90 family are found from bacteria to higher eukaryotes, with homologues in different organelles. The core architecture of Hsp90 is defined by the N-terminal ATP-binding domain, followed by the middle domain and the C-terminal dimerization domain. A long, highly charged linker between the N-terminal domain and the middle domain is a feature characteristic for Hsp90s of eukaryotic organisms. We set out to clarify the function of this linker by studying the effects of deletions in this region in vivo and in vitro. Here we show that increasing deletions in the charged linker region lead to defects ranging from mild temperature-sensitivity to a lethal phenotype. The lethal deletion variants investigated in this study still exhibit ATPase activity. Further, we observed that deletion of the charged linker ultimately causes a loss of Hsp90 regulation by co-chaperones, as the sensitivity for Aha1-mediated ATPase acceleration declines, and binding of p23/Sba1 is lost in non-viable deletion constructs. In vivo client assays additionally demonstrated that the deletion of the linker had a pronounced effect on the ability of Hsp90 to facilitate client activation. A partial reconstruction of the linker-sequence showed that the supplementation by artificial sequences can rescue the functionality of Hsp90 and restore the conformational flexibility of the protein, required for the processing of client proteins.