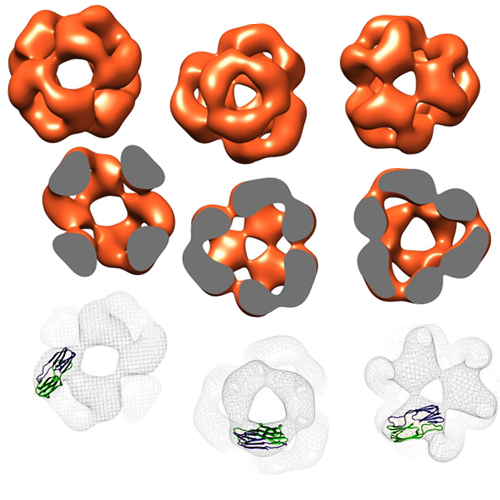
The eye lens chaperone alpha-crystallin forms defined globular assemblies
27-Jul-2009
alpha-Crystallins are molecular chaperones that protect vertebrate eye lens proteins from detrimental protein aggregation. alpha B-Crystallin, 1 of the 2 alpha-crystallin isoforms, is also associated with myopathies and neuropathological diseases. Despite the importance of alpha-crystallins in protein homeostasis, only little is known about their quaternary structures because of their seemingly polydisperse nature. Here, we analyzed the structures of recombinant alpha-crystallins using biophysical methods. In contrast to previous reports, we show that alpha B-crystallin assembles into defined oligomers consisting of 24 subunits. The 3-dimensional (3D) reconstruction of alpha B-crystallin by electron microscopy reveals a spherelike structure with large openings to the interior of the protein. Alpha A-Crystallin forms, in addition to complexes of 24 subunits, also smaller oligomers and large clusters consisting of individual oligomers. This propensity might explain the previously reported polydisperse nature of alpha-crystallin.