A New Class of Rhomboid Protease Inhibitors Discovered by Activity-Based Fluorescence Polarization
22-Aug-2013
PLOS ONE, 2013, DOI: 10.1371/journal.pone.0072307, Volume 8, Issue 8, e72307 published on 22.08.2013
PLOS ONE, online article
PLOS ONE, online article
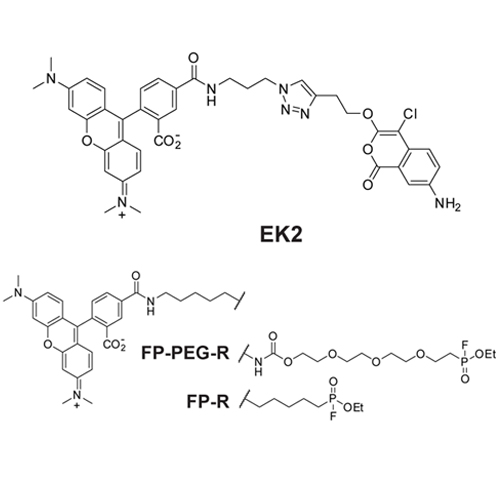
Rhomboids are intramembrane serine proteases that play diverse biological roles, including some that are of potential therapeutical relevance. Up to date, rhomboid inhibitor assays are based on protein substrate cleavage. Although rhomboids have an overlapping substrate specificity, substrates cannot be used universally. To overcome the need for substrates, we developed a screening assay using fluorescence polarization activity-based protein profiling (FluoPol ABPP) that is compatible with membrane proteases. With FluoPol ABPP, we identified new inhibitors for the E. coli rhomboid GlpG. Among these was a structural class that has not yet been reported as rhomboid inhibitors: β-lactones. They form covalent and irreversible complexes with the active site serine of GlpG. The presence of alkyne handles on the β-lactones also allowed activity-based labeling. Overall, these molecules represent a new scaffold for future inhibitor and activity-based probe development, whereas the assay will allow inhibitor screening of ill-characterized membrane proteases.