Aggregation and Reactivity of Organozincate Anions Probed by Electrospray Mass Spectrometry
15-Dec-2008
Organometallics, 2008, DOI: 10.1021/om8007037, published on 15.12.2008
Organometallics, online article
Organometallics, online article
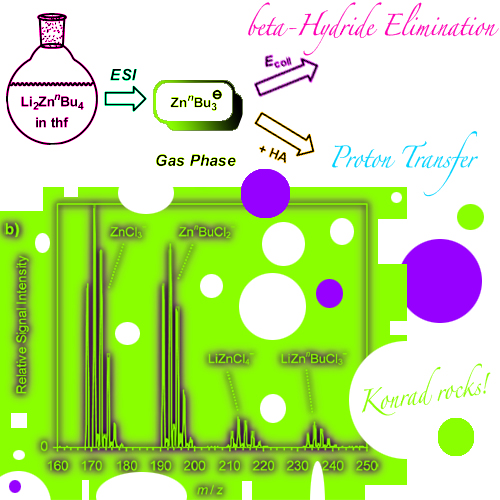
Electrospray ionization (ESI) of mixtures of organolithium compounds and zinc chloride in tetrahydrofuran produced manifold mono- and polynuclear organozincate anions. Formation of the latter is strongly favored by the incorporation of chloride ligands, which apparently adopt bridging binding modes. Analysis of LinBu/ZnCl2 solutions at different concentrations showed that the relative ESI signal intensities for anions in different aggregation states closely correlate with their expected equilibria in solution. Moreover, the uni- and bimolecular gas-phase reactivity of the mass-selected anionic organozincates was studied. Upon collision-induced dissociation, most of these complexes lose a neutral metal fragment, and only the tributylzincates ZnBu3 - react by elimination of alkenes. The tributylzincate complexes were also found to undergo ion-molecule reactions with formic acid. The relative rates for these proton-transfer processes decrease in the series ZnnBu3-, ZnsBu3-, and ZntBu3-, and they also decrease if the butyl groups are substituted for chloride ligands. These trends fully agree with the known solution-phase chemistry of organozinc compounds.