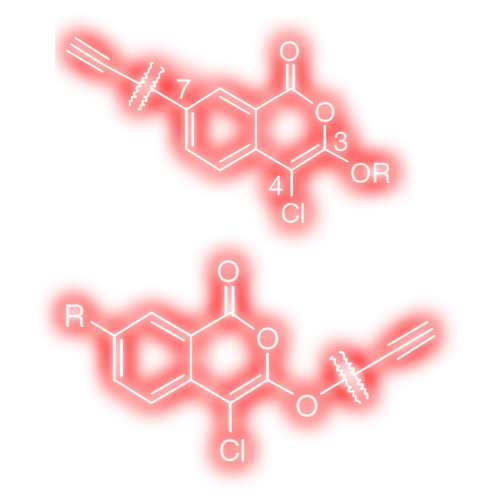
Alkyne derivatives of isocoumarins as clickable activity-based probes for serine proteases
12-Mar-2011
Bioorganic & Medicinal Chemistry, 2011, doi:10.1016/j.bmc.2011.03.014, published on 12.03.2011
Bioorganic & Medicinal Chemistry, online article
Bioorganic & Medicinal Chemistry, online article
Activity-based probes (ABPs) have found increasing use in functional proteomics studies. Recently, ABPs that can be employed in combination with click chemistry gained particular attention due to their flexible application in vitro and in vivo. Moreover, there is a continuous need for new ABPs that target small subsets of enzymes. We here report novel clickable ABPs based on the 4-chloro-isocoumarin (IC) electrophile, a mechanism-based inhibitor scaffold that covalently binds serine proteases. We describe the synthesis of a small library of IC ABPs containing an alkyne function and a set of diverse selectivity elements. The different substituents on the IC structure determine which proteases are bound, showing good correlation with the preferred substrate preferences. The IC ABPs can detect their target proteases in a proteome background in a sensitive manner (down to 0.007% of total protein). Furthermore, we show activity-dependent and selective labeling of endogenous proteases in a tissue proteome. These ICs therefore represent a valuable extension to already existing ABPs for serine proteases and may be instrumental in future elucidation of serine protease functions.