Anticalins: Exploiting a non-Ig scaffold with hypervariable loops for the engineering of binding proteins
21-Jan-2014
FEBSLetter, 2014, DOI: http://dx.doi.org/10.1016/j.febslet.2013.11.006, Volume 588, Issue 2, Pages 213–218 published on 21.01.2014
FEBSLetters, online article
FEBSLetters, online article
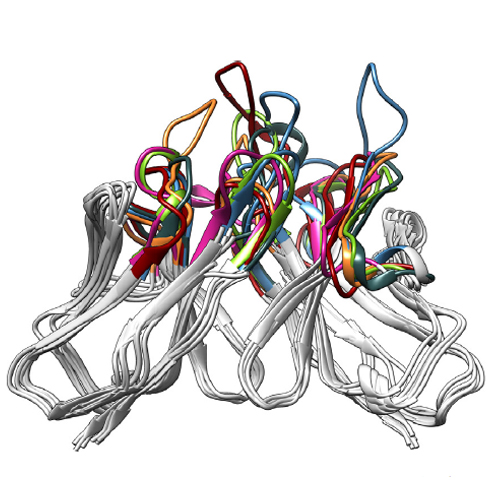
Antibodies, which can recognize a plethora of possible antigens, have been considered as a paradigm of protein engineering performed by nature itself. Lipocalins constitute a distinct family of proteins with functions in ligand binding and transport that occur in many organisms, including man. Like antibodies, lipocalins exhibit a structurally conserved framework – a β-barrel with an attached α-helix – which supports four structurally hypervariable loops forming a cup-shaped binding site. Thus, lipocalins offer an ideal platform for protein engineering to generate novel binding reagents. Using recombinant/synthetic DNA technology and methods of combinatorial library selection, ‘Anticalins’ with prescribed target specificities can be easily generated. Anticalins with picomolar affinities have been developed for three classes of ligands having relevance in basic research and/or medical application: small molecules, peptides, and proteinaceous signalling molecules as well as cell surface receptors. Anticalins derived from human lipocalins have already reached the clinical trial stage. Due to their very small size and simple composition of a single polypeptide chain, which also facilitates the construction of bifunctional fusion proteins, Anticalins promise benefits as a next class of biopharmaceuticals.