Biochemical Analysis of Recombinant AlkJ from Pseudomonas putida Reveals a Membrane-Associated, Flavin Adenine Dinucleotide- Dependent Dehydrogenase Suitable for the Biosynthetic Production of Aliphatic Aldehydes
07-Feb-2014
Appl. Environ. Microbiol., 2014, doi: 10.1128/AEM.04297-13, vol.80, no.8, 2468-2477 published on 07.02.2014
Applied and Environmental Microbiology, online article
Applied and Environmental Microbiology, online article
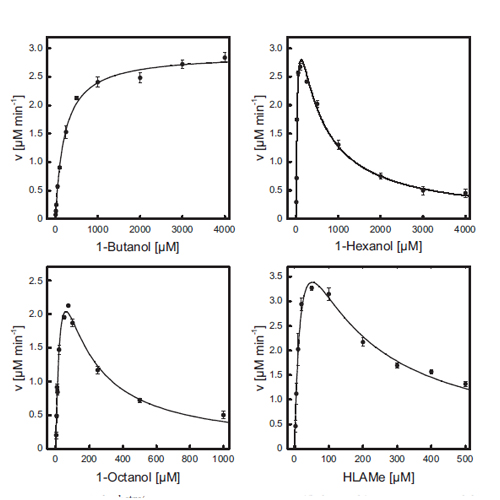
The noncanonical alcohol dehydrogenase AlkJ is encoded on the alkane-metabolizing alk operon of the mesophilic bacterium Pseudomonas putida GPo1. To gain insight into the enzymology of AlkJ, we have produced the recombinant protein in Escherichia coli and purified it to homogeneity using His6 tag affinity and size exclusion chromatography (SEC). Despite synthesis in the cytoplasm, AlkJ was associated with the bacterial cell membrane, and solubilization with n-dodecyl-β-d-maltoside was necessary to liberate the enzyme. SEC and spectrophotometric analysis revealed a dimeric quaternary structure with stoichiometrically bound reduced flavin adenine dinucleotide (FADH2). The holoenzyme showed thermal denaturation at moderate temperatures around 35°C, according to both activity assay and temperature-dependent circular dichroism spectroscopy. The tightly bound coenzyme was released only upon denaturation with SDS or treatment with urea-KBr and, after air oxidation, exhibited the characteristic absorption spectrum of FAD. The enzymatic activity of purified AlkJ for 1-butanol, 1-hexanol, and 1-octanol as well as the n-alkanol derivative ω-hydroxy lauric acid methyl ester (HLAMe) was quantified in the presence of the artificial electron acceptors phenazine methosulfate (PMS) and 2,6-dichlorophenolindophenol (DCPIP), indicating broad substrate specificity with the lowest activity on the shortest alcohol, 1-butanol. Furthermore, AlkJ was able to accept as cosubstrates/oxidants the ubiquinone derivatives Q0 and Q1, also in conjunction with cytochrome c, which suggests coupling to the bacterial respiratory chain of this membrane-associated enzyme in its physiological environment. Using gas chromatographic analysis, we demonstrated specific biocatalytic conversion by AlkJ of the substrate HLAMe to the industrially relevant aldehyde, thus enabling the biotechnological production of 12-amino lauric acid methyl ester via subsequent enzymatic transamination.