Design and Characterization of a Modular Membrane Protein Anchor to Functionalize the Moss Physcomitrella patens with Extracellular Catalytic and/or Binding Activities
19-Dec-2014
ACS Synth. Biol., 3 (12), pp 990–994, DOI: 10.1021/sb5000302
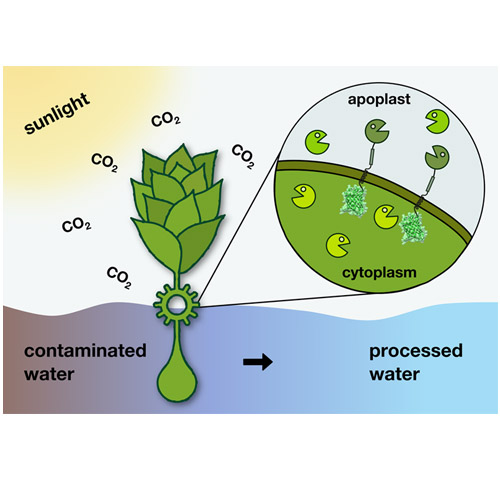
Heterologous enzymes and binding proteins were secreted by the moss Physcomitrella patens or anchored extracellularly on its cell membrane in order to functionalize the apoplast as a biochemical reaction compartment. This modular membrane anchoring system utilizes the signal peptide and the transmembrane segment of the somatic embryogenesis receptor-like kinase (SERK), which were identified in a comprehensive bioinformatic analysis of the P. patens genome. By fusing the soluble enzyme NanoLuc luciferase to the signal peptide, its secretion capability was confirmed in vivo. The membrane localization of hybrid proteins comprising the SERK signal peptide, NanoLuc or other functional modules, the SERK transmembrane anchor, and a C-terminal GFP reporter was demonstrated using fluorescence microscopy as well as site-specific proteolytic release of the extracellular enzyme domain. Our membrane anchoring system enables the expression of various functional proteins in the apoplast of P. patens, empowering this photoautotrophic organism for biotechnological applications.