Elucidation of the a-Keto-Aldehyde Binding Mechanism: A Lead Structure Motif for Proteasome Inhibition
10-Jan-2011
Angewandte Chemie, 2011, 50, 2, 542 - 44 published on 10.01.2011
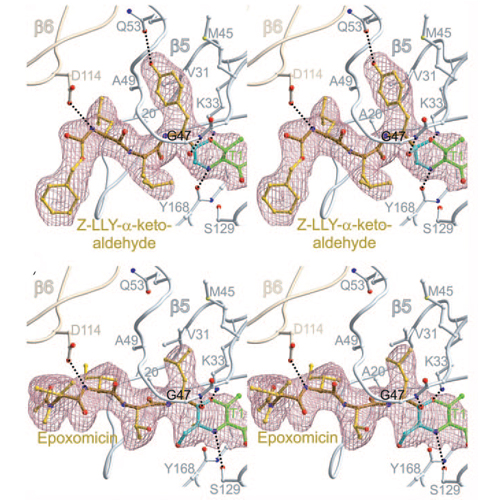
The proteasome's participation in essential biological processes such as stress response, cell proliferation, apoptosis, and antigen presentation has been well established.[1] It is, therefore, not surprising that academia and the pharmaceutical industry have made efforts to develop a range of small synthetic inhibitors against this proteolytic molecular machine (see Scheme SS1 in the Supporting Information for examples).[2] An overall structural comparison of some wellcharacterized inhibitors[3] implies that most of these compounds form a covalent bond with the N-terminal nucleophilic threonine (Thr1)[4] located at the active sites in the two inner heptameric b rings of the 20S proteasome, termed beta1, beta2, and beta5 according to the subunit of their origin.