Enhanced in Vivo Efficacy of a Type I Interferon Superagonist with Extended Plasma Half-life in a Mouse Model of Multiple Sclerosis
05-Sep-2014
THE JOURNAL OF BIOLOGICAL CHEMISTRY, 2014, DOI 10.1074/jbc.M114.602474, VOL. 289, NO. 42, pp. 29014–29029 published on 05.09.2014
JBC, online article
JBC, online article
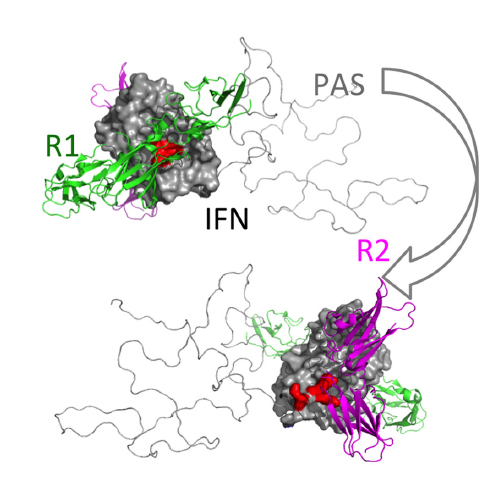
IFNβ is a common therapeutic option to treat multiple sclerosis (MS). It is unique amongst the family of type I IFNs in that it binds to the interferon receptors with high affinity, conferring exceptional biological properties. We have previously reported the generation of an interferon superagonist (dubbed YNSα8) that is built on the backbone of a low affinity IFNα, but modified to exhibit higher receptor affinity than even for IFNβ. Here, YNSα8 was fused with a 600-residue hydrophilic, unstructured N-terminal polypeptide chain comprising Proline, Alanine and Serine (PAS) in order to prolong its plasma half-life via "PASylation". PAS-YNSα8 exhibited a 10-fold increased half-life in both pharmacodynamic and pharmacokinetic assays in a transgenic mouse model harboring the human receptors, notably without any detectable loss in biological potency or bioavailability. This long-life superagonist conferred significantly improved protection from MOG35-55-induced experimental autoimmune encephalomyelitis (EAE) compared to IFNβ, despite being injected with a 4-fold less frequency and at an overall 16-fold lower dosage. These data were corroborated by FACS measurements showing a decrease of CD11b+ve / CD45hi myeloid lineage cells detectable in the central nervous system (CNS), as well as a decrease in IBA+ve cells in spinal cord sections determined by immunohistochemistry for PAS-YNSα8 treated animals. Importantly, PAS-IFNα8 did not induce antibodies upon repeated administration, and its biological efficacy remained unchanged after 21 days of treatment. A striking correlation between increased levels of CD274 (PD-L1) transcripts from spleen-derived CD4+ cells and improved EAE clinical response was observed, indicating that at least in this mouse model of MS, CD274 may serve as a biomarker to predict effectiveness of IFN therapy to treat this complex disease.